Biosurfactant-induced PFAS Leaching from Aqueous Film-Forming Foam (AFFF) Impacted Soil
DOI:
https://doi.org/10.69631/7v8xmn98Keywords:
PFOS, Perfluorooctane sulfonic acid, Rhamnolipid, Soil flushing, Column testingAbstract
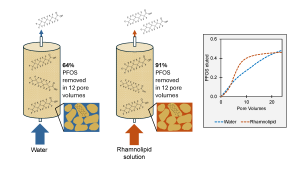
The development of sustainable per- and polyfluoroalkyl (PFAS) remediation techniques is critical for the removal of contaminants from soil and water at sites impacted by aqueous film-forming foam (AFFF). This study is the first to explore the feasibility of flushing PFAS with a rhamnolipid biosurfactant solution using column testing and soil from an AFFF-contaminated site. Soil was flushed by either tap water alone or a 0.005% rhamnolipid solution. The PFAS concentrations in eluate and mass balances were compared for each test. In the first 12 pore volumes, 91% of the total perfluorooctane sulfonic acid (PFOS) flushed by the rhamnolipid solution was removed, while only 64% of PFOS was flushed in that time by tap water alone. Phosphate leached from soil and PFOS measured in the same eluate had similar concentration patterns, suggesting competitive sorption occurs with negatively charged phosphate, PFOS, and the anionic biosurfactant rhamnolipid. The flushing tests also show that there is no significant difference in flushing with a biosurfactant for PFAS compounds other than PFOS. Transport modeling confirmed that PFOS retardation (R-value) was lower with the rhamnolipid solution (R = 9.76) than with tap water (R = 22.3), which indicates that it is more efficient at removing PFOS from soil than water alone. This concept study provides insight into the release of PFAS compounds from a real contaminated saturated soil containing organic carbon, clay, and a complex mixture of PFAS. It shows promising first results for the use of biosurfactants as a sustainable flushing strategy.
Downloads
References
1. Abou-Khalil, C., Sarkar, D., Braykaa, P., & Boufadel, M. C. (2022). Mobilization of per- and polyfluoroalkyl substances (PFAS) in soils: A review. Current Pollution Reports, 8(4), 422–444. https://doi.org/10.1007/s40726-022-00241-8
2. Abraham, J. E. F., Mumford, K. G., Patch, D. J., & Weber, K. P. (2022). Retention of PFOS and PFOA mixtures by trapped gas bubbles in porous media. Environmental Science & Technology, 56(22), 15489–15498. https://doi.org/10.1021/acs.est.2c00882
3. Barrow, N. J. (2015). A mechanistic model for describing the sorption and desorption of phosphate by soil. European Journal of Soil Science, 66(1), 9–18. https://doi.org/10.1111/ejss.12198_2
4. Brusseau, M. L. (2020). Simulating PFAS transport influenced by rate-limited multi-process retention. Water Research, 168, 115179. https://doi.org/10.1016/j.watres.2019.115179
5. Brusseau, M. L., Guo, B., Huang, D., Yan, N., & Lyu, Y. (2021). Ideal versus nonideal transport of PFAS in unsaturated porous media. Water Research, 202, 117405. https://doi.org/10.1016/j.watres.2021.117405
6. Brusseau, M. L., Lyu, Y., Yan, N., & Guo, B. (2020). Low-concentration tracer tests to measure air-water interfacial area in porous media. Chemosphere, 250, 126305. https://doi.org/10.1016/j.chemosphere.2020.126305
7. Brusseau, M. L., Yan, N., Van Glubt, S., Wang, Y., Chen, W., et al. (2019). Comprehensive retention model for PFAS transport in subsurface systems. Water Research, 148, 41–50. https://doi.org/10.1016/j.watres.2018.10.035
8. Chen, H., Reinhard, M., Nguyen, T. V., You, L., He, Y., & Gin, K. Y.-H. (2017). Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment. Environmental Pollution, 227, 397–405. https://doi.org/10.1016/j.envpol.2017.04.091
9. D’Agostino, L. A., & Mabury, S. A. (2014). Identification of novel fluorinated surfactants in aqueous film forming foams and commercial surfactant concentrates. Environmental Science & Technology, 48(1), 121–129. https://doi.org/10.1021/es403729e
10. Dai, M., Yan, N., & Brusseau, M. L. (2023). Potential impact of bacteria on the transport of PFAS in porous media. Water Research, 243, 120350. https://doi.org/10.1016/j.watres.2023.120350
11. De Rijk, V., Buma, J., Veldkamp, H., & Zech, A. (2025). Predicting saturated hydraulic conductivity from particle size distributions using machine learning. Stochastic Environmental Research and Risk Assessment, 39(2), 423–435. https://doi.org/10.1007/s00477-024-02861-6
12. De Silva, A. O., Armitage, J. M., Bruton, T. A., Dassuncao, C., Heiger-Bernays, W., et al. (2020). PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environmental Toxicology and Chemistry, 40(3), 631–657. https://doi.org/10.1002/etc.4935
13. Dupla, X., Gondret, K., Sauzet, O., Verrecchia, E., & Boivin, P. (2021). Changes in topsoil organic carbon content in the Swiss leman region cropland from 1993 to present. Insights from large scale on-farm study. Geoderma, 400, 115125. https://doi.org/10.1016/j.geoderma.2021.115125
14. Edmonds, D. A., Caldwell, R. L., Brondizio, E. S., & Siani, S. M. O. (2020). Coastal flooding will disproportionately impact people on river deltas. Nature Communications, 11(1), 4741. https://doi.org/10.1038/s41467-020-18531-4
15. Farias, C. B. B., Almeida, F. C. G., Silva, I. A., Souza, T. C., Meira, H. M., et al. (2021). Production of green surfactants: Market prospects. Electronic Journal of Biotechnology, 51, 28–39. https://doi.org/10.1016/j.ejbt.2021.02.002
16. Gnesda, W. R., Draxler, E. F., Tinjum, J., & Zahasky, C. (2022). Adsorption of PFAAs in the vadose zone and implications for long-term groundwater contamination. Environmental Science & Technology, 56(23), 16748–16758. https://doi.org/10.1021/acs.est.2c03962
17. Gudiña, E. J., Rodrigues, A. I., Alves, E., Domingues, M. R., Teixeira, J. A., & Rodrigues, L. R. (2015). Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresource Technology, 177, 87–93. https://doi.org/10.1016/j.biortech.2014.11.069
18. Guelfo, J. L., & Higgins, C. P. (2013). Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environmental Science & Technology, 47(9), 4164–4171. https://doi.org/10.1021/es3048043
19. Guo, B., & Brusseau, M. L. (2024). Challenges and opportunities for porous media research to address PFAS groundwater contamination. InterPore Journal, 1(2), ipj240824-2. https://doi.org/10.69631/ipj.v1i2nr35
20. Guo, B., Saleem, H., & Brusseau, M. L. (2023). Predicting interfacial tension and adsorption at fluid–fluid interfaces for mixtures of PFAS and/or hydrocarbon surfactants. Environmental Science & Technology, 57(21), 8044–8052. https://doi.org/10.1021/acs.est.2c08601
21. Guo, B., Zeng, J., & Brusseau, M. L. (2020). A mathematical model for the release, transport, and retention of per‐ and polyfluoroalkyl substances (PFAS) in the vadose zone. Water Resources Research, 56(2), e2019WR026667. https://doi.org/10.1029/2019WR026667
22. He, K., Feerick, A., Jin, H., Batista Andrade, J. A., Duarte Batista, M., et al. (2024). Retention of per- and polyfluoroalkyl substances by syringe filters. Environmental Chemistry Letters, 22(4), 1569–1579. https://doi.org/10.1007/s10311-024-01718-2
23. Helmy, Q., Gustiani, S., & Mustikawati, A. (2020). Application of rhamnolipid biosurfactant for bio-detergent formulation. IOP Conference Series: Materials Science and Engineering, 823(1), 012014. https://doi.org/10.1088/1757-899X/823/1/012014
24. Høisæter, Å., Pfaff, A., & Breedveld, G. D. (2019). Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. Journal of Contaminant Hydrology, 222, 112–122. https://doi.org/10.1016/j.jconhyd.2019.02.010
25. Houtz, E. F., Higgins, C. P., Field, J. A., & Sedlak, D. L. (2013). Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental Science & Technology, 47(15), 8187–8195. https://doi.org/10.1021/es4018877
26. Hu, X. C., Andrews, D. Q., Lindstrom, A. B., Bruton, T. A., Schaider, L. A., et al. (2016). Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. Drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environmental Science & Technology Letters, 3(10), 344–350. https://doi.org/10.1021/acs.estlett.6b00260
27. Huang, D., Saleem, H., Guo, B., & Brusseau, M. L. (2022). The impact of multiple-component PFAS solutions on fluid-fluid interfacial adsorption and transport of PFOS in unsaturated porous media. Science of The Total Environment, 806, 150595. https://doi.org/10.1016/j.scitotenv.2021.150595
28. ISO. (2019). ISO 21268-3:2019. Soil Quality - Leaching Procedures for Subsequent Chemical and Ecotoxicological Testing of Soil and Soil-Like Materials - Part 3: Up-flow Percolation Test. International Organization for Standardization. https://www.iso.org/standard/68252.html
29. ITRC. (2018). Environmental Fate and Transport for Per- and Polyfluoroalkyl Substances. PFAS Fact Sheets 5. Interstate Technology Regulatory Council. https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://documents.dps.ny.gov/public/Common/ViewDoc.aspx%3FDocRefId%3D%257B5BA26C14-0AA1-4A81-A04F-7D40DC33595B%257D&ved=2ahUKEwjptPXN_6iQAxXv-AIHHbP6EQcQFnoECBoQAQ&usg=AOvVaw3-fSnVcC0XtmFFEhixZqBC
30. ITRC. (2023 September). Sampling Precautions and Laboratory Analytical Methods for Per- and Polyfluoroalkyl Substances (PFAS). PFAS Fact Sheets. Interstate Technology Regulatory Council. https://pfas-1.itrcweb.org/wp-content/uploads/2022/09/Sampling_and_Lab_PFAS_Fact-Sheet_082522_508.pdf
31. Ji, Y., Yan, N., Brusseau, M. L., Guo, B., Zheng, X., et al. (2021). Impact of a hydrocarbon surfactant on the retention and transport of perfluorooctanoic acid in saturated and unsaturated porous media. Environmental Science & Technology, 55(15), 10480–10490. https://doi.org/10.1021/acs.est.1c01919
32. Kabiri, S., Tucker, W., Navarro, D. A., Bräunig, J., Thompson, K., et al. (2022). Comparing the leaching behavior of per- and polyfluoroalkyl substances from contaminated soils using static and column leaching tests. Environmental Science & Technology, 56(1), 368–378. https://doi.org/10.1021/acs.est.1c06604
33. Krumina, L., Kenney, J. P. L., Loring, J. S., & Persson, P. (2016). Desorption mechanisms of phosphate from ferrihydrite and goethite surfaces. Chemical Geology, 427, 54–64. https://doi.org/10.1016/j.chemgeo.2016.02.016
34. Kuyukina, M. S., Ivshina, I. B., Makarov, S. O., Litvinenko, L. V., Cunningham, C. J., & Philp, J. C. (2005). Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environment International, 31(2), 155–161. https://doi.org/10.1016/j.envint.2004.09.009
35. Liao, S., Arshadi, M., Woodcock, M. J., Saleeba, Z. S. S. L., Pinchbeck, D., et al. (2022). Influence of residual nonaqueous-phase liquids (NAPLs) on the transport and retention of perfluoroalkyl substances. Environmental Science & Technology, 56(12), 7976–7985. https://doi.org/10.1021/acs.est.2c00858
36. Liu, M., Munoz, G., Vo Duy, S., Sauvé, S., & Liu, J. (2022). Per- and polyfluoroalkyl substances in contaminated soil and groundwater at airports: A Canadian case study. Environmental Science & Technology, 56(2), 885–895. https://doi.org/10.1021/acs.est.1c04798
37. Luft, C. M., Schutt, T. C., & Shukla, M. K. (2022). Properties and mechanisms for PFAS adsorption to aqueous clay and humic soil components. Environmental Science & Technology, 56(14), 10053–10061. https://doi.org/10.1021/acs.est.2c00499
38. Lyu, X., Li, Z., Wang, D., Zhang, Q., Gao, B., et al. (2022). Transport of perfluorooctanoic acid in unsaturated porous media mediated by SDBS. Journal of Hydrology, 607, 127479. https://doi.org/10.1016/j.jhydrol.2022.127479
39. Lyu, Y., & Brusseau, M. L. (2020). The influence of solution chemistry on air-water interfacial adsorption and transport of PFOA in unsaturated porous media. Science of The Total Environment, 713, 136744. https://doi.org/10.1016/j.scitotenv.2020.136744
40. Madsen, J. K., Pihl, R., M ller, A. H., Madsen, A. T., Otzen, D. E., & Andersen, K. K. (2015). The anionic biosurfactant rhamnolipid does not denature industrial enzymes. Frontiers in Microbiology, 6. https://doi.org/10.3389/fmicb.2015.00292
41. Maimaiti, A., Deng, S., Meng, P., Wang, W., Wang, B., et al. (2018). Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chemical Engineering Journal, 348, 494–502. https://doi.org/10.1016/j.cej.2018.05.006
42. Maizel, A. C., Shea, S., Nickerson, A., Schaefer, C., & Higgins, C. P. (2021). Release of per- and polyfluoroalkyl substances from aqueous film-forming foam impacted soils. Environmental Science & Technology, 55(21), 14617–14627. https://doi.org/10.1021/acs.est.1c02871
43. Naka, A., Yasutaka, T., Sakanakura, H., Kalbe, U., Watanabe, Y., et al. (2016). Column percolation test for contaminated soils: Key factors for standardization. Journal of Hazardous Materials, 320, 326–340. https://doi.org/10.1016/j.jhazmat.2016.08.046
44. New risk limits for PFAS in surface water | RIVM. (2022 September 26). National Institute for Public Health and the Environment. https://www.rivm.nl/en/news/new-risk-limits-for-PFAS-in-surface-water
45. Nguyen, T. T., Youssef, N. H., McInerney, M. J., & Sabatini, D. A. (2008). Rhamnolipid biosurfactant mixtures for environmental remediation. Water Research, 42(6–7), 1735–1743. https://doi.org/10.1016/j.watres.2007.10.038
46. Niarchos, G., Ahrens, L., Kleja, D. B., & Fagerlund, F. (2022). Per- and polyfluoroalkyl substance (PFAS) retention by colloidal activated carbon (CAC) using dynamic column experiments. Environmental Pollution, 308, 119667. https://doi.org/10.1016/j.envpol.2022.119667
47. Nickerson, A., Maizel, A. C., Schaefer, C. E., Ranville, J. F., & Higgins, C. P. (2023). Effect of geochemical conditions on PFAS release from AFFF-impacted saturated soil columns. Environmental Science: Processes & Impacts, 25(3), 405–414. https://doi.org/10.1039/D2EM00367H
48. Onaizi, S. A. (2018). Dynamic surface tension and adsorption mechanism of surfactin biosurfactant at the air–water interface. European Biophysics Journal, 47(6), 631–640. https://doi.org/10.1007/s00249-018-1289-z
49. Reijneveld, A., Van Wensem, J., & Oenema, O. (2009). Soil organic carbon contents of agricultural land in the Netherlands between 1984 and 2004. Geoderma, 152(3–4), 231–238. https://doi.org/10.1016/j.geoderma.2009.06.007
50. Senevirathna, S. T. M. L. D., Mahinroosta, R., Li, M., & KrishnaPillai, K. (2021). In situ soil flushing to remediate confined soil contaminated with PFOS- an innovative solution for emerging environmental issue. Chemosphere, 262, 127606. https://doi.org/10.1016/j.chemosphere.2020.127606
51. Shojaei, M., Kumar, N., Chaobol, S., Wu, K., Crimi, M., & Guelfo, J. (2021). Enhanced recovery of per- and polyfluoroalkyl substances (PFASs) from impacted soils using heat activated persulfate. Environmental Science & Technology, 55(14), 9805–9816. https://doi.org/10.1021/acs.est.0c08069
52. Simunek, J., M. van Genuchten, M. Sejna, N. Toride, and F. J. Leij. (1999). The STANMOD Computer Software for Evaluating Solute Transport in Porous Media Using Analytical Solutions of Convection-Dispersion Equation. Research {Report} IGWMC - TPS - 71. Golden, Colorado: International Ground Water Modeling Center, Colorado School of Mines. https://www.pc-progress.com/Documents/Jirka/Stanmod.pdf
53. Smeck, Neil E. 1973. “Phosphorus: An Indicator of Pedogenetic Weathering Processes.” Soil Science 115 (3): 199–206. https://journals.lww.com/soilsci/abstract/1973/03000/phosphorus__an_indicator_of_pedogenetic_weathering.5.aspx
54. Suhandono, S., Kusuma, S. H., & Meitha, K. (2021). Characterization and production of rhamnolipid biosurfactant in recombinant escherichia coli using autoinduction medium and palm oil mill effluent. Brazilian Archives of Biology and Technology, 64, e21200301. https://doi.org/10.1590/1678-4324-2021200301
55. Toride, N., F. J. Leij, and M. van Genuchten. (1995). The CXTFIT Code for Estimating Transport Parameters from Laboratory or Field Tracer Experiments. Research Report 137. Riverside, California: U.S. Salinity Lab., USDA, ARS. https://www.ars.usda.gov/arsuserfiles/20360500/pdf_pubs/P1444.pdf
56. Tsou, K., Antell, E., Duan, Y., Olivares, C. I., Yi, S., Alvarez-Cohen, L., & Sedlak, D. L. (2023). Improved total oxidizable precursor assay for quantifying polyfluorinated compounds amenable to oxidative conversion to perfluoroalkyl carboxylic acids. ACS ES&T Water, 3(9), 2996–3003. https://doi.org/10.1021/acsestwater.3c00224
57. US EPA. (2021 August). Draft Method 1633 analysis of per-and polyfluoroalkyl substances (PFAS) in aqueous, solid, biosolids, and tissue samples by LC-MS/MS. United States Environmental Protection Agency. https://www.epa.gov/system/files/documents/2021-09/method_1633_draft_aug-2021.pdf
58. US EPA. (2025 May 5). SW-846 Test Method 1314: Liquid-solid Partitioning as a Function of Liquid-Solid Ratio for Constituents in Solid Materials Using an up-Flow Percolation Column Procedure. United States Environmental Protection Agency. https://www.epa.gov/hw-sw846/sw-846-test-method-1314-liquid-solid-partitioning-function-liquid-solid-ratio-constituents
59. US EPA. (2022 December). IRIS Toxicological Review of Perfluorobutanoic Acid (PFBA, CASRN 37522-4) and Related Salts. EPA/635/R-22/277Fa. United States Environmental Protection Agency. https://iris.epa.gov/static/pdfs/0701tr.pdf
60. US EPA. (2024, November 26). Our current understanding of the human health and environmental risks of PFAS [Overviews and Factsheets]. https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas
61. van Asseldonk, I. 2021. Nader Bodemonderzoek Blusplaats Munsterlaan Te Utrecht. [Further soil investigation at the Munsterlaan fire site in Utrecht]. Technical Report. 213044. BK Engineers, The Netherlands.
62. Van Glubt, S., & Brusseau, M. L. (2021). Contribution of nonaqueous-phase liquids to the retention and transport of per and polyfluoroalkyl substances (PFAS) in porous media. Environmental Science & Technology, 55(6), 3706–3715. https://doi.org/10.1021/acs.est.0c07355
63. Woudneh, M. B., Chandramouli, B., Hamilton, C., & Grace, R. (2019). Effect of sample storage on the quantitative determination of 29 PFAS: Observation of analyte interconversions during storage. Environmental Science & Technology, 53(21), 12576–12585. https://doi.org/10.1021/acs.est.9b03859
64. Yasutaka, T., Naka, A., Sakanakura, H., Kurosawa, A., Inui, T., et al. (2017). Reproducibility of up-flow column percolation tests for contaminated soils. PLOS ONE, 12(6), e0178979. https://doi.org/10.1371/journal.pone.0178979
Downloads
Additional Files
Published
Data Availability Statement
Data and protocols are available in the Supplementary Material.
Issue
Section
License
Copyright (c) 2025 Sophie R. Hibben, Alraune Zech, Bas van der Grift, Jacco Koekoek, Sicco Brandsma, Johan van Leeuwen

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Unless otherwise stated above, this is an open access article published by InterPore under either the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Article metadata are available under the CCo license.













